Electromagnets & Motors
By Alan Zahn Overview: This lesson describes how to make simple electromagnets and a motor that is powered by a solar panel. Essential Question: How can electricity be used to cause magnetism? Background: A magnet is an object that produces a magnetic field. We are already familiar with certain types of magnetic fields such as the Earth’s magnetic field that a compass uses to point north, or refrigerator magnets that use magnetic fields to stick. Magnets have uses beyond being interesting toys and sticking things on metal, and one of the most widespread uses of magnets in industry is in electric motors. In electric motors, the magnet is used to...
Phosphorescent Decay
Overview: In this activity you will measure the phosphorescence over time of a glow-in-the-dark paint following excitation by a flash of light. Then the data is transferred to Excel to find the best fit curve and its equation. This provides an insight into reaction order and methods for studying emissive processes. phosphorescent decay lesson Essential Question: What reaction order do you predict the phosphorescent emission from glow in the dark paper will follow? What affect will temperature have on the phosphorescent decay pattern? Background: Fluorescence vs Phosphorescence Many materials exhibit fluorescence when they are excited by a sufficiently energetic light source. In an excited material a population of electrons and holes is...
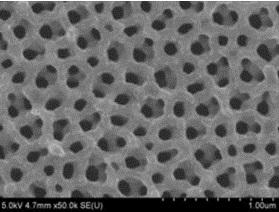
Mathematics of Porous Materials
Nanotechnology provides new ways to make novel materials. Surface area is a key feature of devices that utilize chemical reactions. Mathematics gives us a way to analyze the surface to volume ratio. Watch this video to see how the same amount of material can be processed to create an extremely high surface area. This is useful for constructing high capacity battery electrodes. Download Powerpoint version of above presentation Photograph by Alexander Kozen, ANSLab University of Maryland Porous Materials for CEI Revision 3 Challenge: See if you can create a spreadsheet that models the effect of decreasing particle size on surface to volume ratio for a bulk material. (hint: calculate the...
Fuzzy Molecule Challenge
[vc_row][vc_column][vc_column_text]Introduction Use pipe cleaners and pom poms to make models of common molecules. Molecules are combinations of atoms which share electrons to form covalent chemical bonds. The compounds formed have different properties than the atoms they are made from. Materials Pipecleaner and pom poms - Amazon - $11.98 Directions • Balls represent atoms. In the diagrams below oxygen is red, hydrogen is white, carbon is gray, and nitrogen is blue. • Colored Pom Poms are different kinds of atoms. • Pipe cleaners represent bonds. • Atoms have different numbers of bonds they can form. • Twist the pipe cleaners around the pom poms to make models of these molecules. • Variation: use marshmallows and toothpicks...
Polymers
[vc_row][vc_column][mkd_section_title title="Polymers" title_size="large" title_color="" title_text_align="" margin_bottom="" width=""][vc_column_text]By Monica Esopi The intention of this lesson is to learn background about polymer materials and their applications, and to explore these materials through hands-on activities (making slime and bouncy balls). Students will be able to make their own polymers and explore their properties. These activities can be done individually, or in pairs or groups. Students will make slime to explore cross-linking, and then make bouncy balls to see the impact of a thickening agent.[/vc_column_text][vc_empty_space height="30px"][vc_hoverbox image="18213" primary_title="" primary_align="left" hover_title="QUESTION" shape="square" el_width="30" align="left"]How can simple molecules be joined together in chains or networks to make a substance with different...
Bubble Raft Crystal Model
[vc_row][vc_column][mkd_section_title title="Bubble Raft Crystal Model" title_size="large" title_color="" title_text_align="" margin_bottom="" width=""][vc_column_text]In this demonstration / lab students use uniform bubbles floating on water to model the formation and organization of atoms in crystals (Bubleraft Lesson)[/vc_column_text][vc_empty_space height="30px"][vc_hoverbox image="18209" primary_title="" primary_align="left" hover_title="QUESTION" shape="square" el_width="30" align="left"]Can we use bubbles floating on water to model the organization of atoms forming a crystal?[/vc_hoverbox][vc_empty_space height="40px"][mkd_accordion style="boxed_toggle" el_class="GLOWING COLORS"][mkd_accordion_tab icon_pack="" title="Background"][vc_column_text]Self-assembly is the idea that particles can organize themselves into the complex structures with a high amount of order. Closely packed balls of the same size will eventually sort themselves into tightly packed rows that look like the atoms arranged in crystals. At...
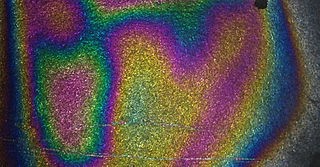
Rainbow Bookmarker
[vc_row][vc_column][mkd_section_title title="Rainbow Bookmarks" title_size="large" title_color="" title_text_align="" margin_bottom="" width=""][vc_column_text]In this activity, students trap an extremely thin layer of clear nail polish which causes interference of light waves making a rainbow layer on a black bookmark. PDF[/vc_column_text][vc_empty_space height="30px"][vc_hoverbox image="18203" primary_title="" primary_align="left" hover_title="QUESTION" shape="square" el_width="30" align="left"]What happens when light reflects off thin layers of materials?[/vc_hoverbox][vc_empty_space height="40px"][mkd_accordion style="boxed_toggle" el_class="GLOWING COLORS"][mkd_accordion_tab icon_pack="" title="Background"][vc_column_text]The reason the rainbow is seen is because the layer of clear nail polish is so thin that it reflects light in different wavelengths. Slight difference in thickness cause light waves of different length to interfere with each other-- sometimes cancelling and sometimes reinforcing. Other examples of...
Absorption and Fluorescence with USB Spectrometer
[vc_row][vc_column][vc_column_text] Overview: [caption id="attachment_4862" align="alignright" width="297"] A USB spectrometer measures light that passes through a solution in the cuvette.[/caption] Students explore how different materials absorb and emit light, then measure spectra with a desktop spectrometer. Essential Question: How do we measure the absorption and emission of light? Background: Light travels from here to there like a really fast bullet. When we see a light turn on, there are particles of light called photons that travel from the lamp to your eye. Light goes so fast that in one second, light travels 180,000 miles! That's traveling across the US 72 times in one second! All light is made of photons, when a...
Glowing Colors
[vc_row][vc_column][vc_column_text]Students explore how different materials absorb and emit light of different colors[/vc_column_text][vc_empty_space height="30px"][vc_hoverbox image="18248" primary_title="" primary_align="left" hover_title="QUESTION" shape="square" el_width="30" align="left"]How can materials make light of different colors?[/vc_hoverbox][vc_empty_space height="40px"][mkd_accordion style="boxed_toggle" el_class="GLOWING COLORS"][mkd_accordion_tab icon_pack="" title="Background"][vc_column_text]White light is composed of lights of different colors. Each color is carried by a light moving as a wave. Different materials reflect light of different colors, or they absorb light of other colors. If an object appears red, it because all the other colors besides red are absorbed and only red light is reflected. Some materials create light when they are energized by light, electricity, heat or by chemical reactions. This...