Nanocrystalline Dye Solar Cell
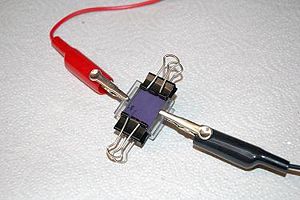
[vc_row][vc_column][vc_column_text] Overview: Students create a dye sensitized solar cell that can generate a small current using nanocrystalline TiO2 and berry juice. Essential Question: How can we make a device which captures solar energy to produce electricity? Background: [caption id="attachment_10755" align="alignright" width="300"] A finished cell is held together with clips.[/caption] This dye-sensitized solar cell, also known as a Grätzel cell, uses a thin film of titanium dioxide which has been ground to a fine powder (nanocrystalline) to increase its reactive surface area. The TiO2 is sandwiched between two glass slides that are coated with conductive and transparent indium tin oxide (ITO). The TiO2 is impregnated with some kind of colored dye, in this...
Mathematics of Porous Materials
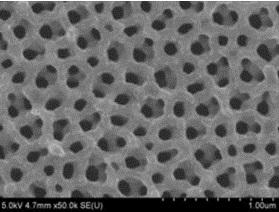
Nanotechnology provides new ways to make novel materials. Surface area is a key feature of devices that utilize chemical reactions. Mathematics gives us a way to analyze the surface to volume ratio. Watch this video to see how the same amount of material can be processed to create an extremely high surface area. This is useful for constructing high capacity battery electrodes. Download Powerpoint version of above presentation Photograph by Alexander Kozen, ANSLab University of Maryland Porous Materials for CEI Revision 3 Challenge: See if you can create a spreadsheet that models the effect of decreasing particle size on surface to volume ratio for a bulk material. (hint: calculate the...
Aluminum Air Battery
[vc_row][vc_column][mkd_section_title title="Aluminum Air Battery" title_size="large" title_color="" title_text_align="" margin_bottom="" width=""][vc_column_text]Storing energy is one of the biggest challenges facing the scaling up of clean energy technologies. The goal of this activity is to allow students to design and build a battery using their understanding of oxidation and reduction reactions. Students will use everyday materials, including aluminum foil, salt water, charcoal, and copper foil, to build a non-rechargeable battery cell capable of powering an LED. Students should be familiar with balancing equations using half-reactions. Essential Questions: Can energy be captured and stored? How can we capture the energy released from a chemical reaction to produce useful electrical power?[/vc_column_text][/vc_column][/vc_row][vc_row][vc_column][mkd_accordion style="toggle"][mkd_accordion_tab icon_pack=""...
Water Model of Electricity
Created by Nathan Wilson for the University of Washington Clean Energy Institute Overview: The goal of this activity is to allow students to apply their knowledge of Ohm’s law to a more intuitive and visual system: water flowing through tubes. The total time for this activity is around 30 minutes. Students are assumed to be familiar Ohm’s law (V/R=I) where V is volts, R is resistance, and I is current, as well as the formula for calculating the resistance of a resistor (R=ρl/A). where p is resistivity in ohms/meter, l is length, A is area of the conductor. Essential Question: How can we model the behavior of electricity...
A Battery from Household Chemicals
Background We use batteries to power lots of different things, from TV remotes to computers. Some cars even run on batteries instead of gasoline! So where does all that power come from? All batteries, big and small, get their power from chemical reactions happening inside of them. When certain types of chemicals react, they give up electrons which we harness to produce electrical power. When you turn on a laptop computer, or press a button on a TV remote, you’re making chemistry happen! While the two ends of the battery are connected, and electricity is flowing, the chemicals can react until they’re all used up,...