Each summer, community college teachers work with UW faculty to bring new clean energy research to undergraduate labs.
February 18, 2020
Founded in 2016 with support from the National Science Foundation (NSF), the Clean Energy Institute’s Research Experience for Teachers (RET) summer program places community college instructors in UW research groups. The instructors work closely with UW faculty and graduate students for six weeks to develop a clean energy curriculum and experiments to bring back to their undergraduate students.
Below, meet two recent alums of the program.
James Patterson, chemistry faculty at North Seattle College
Exploring next-generation solar energy
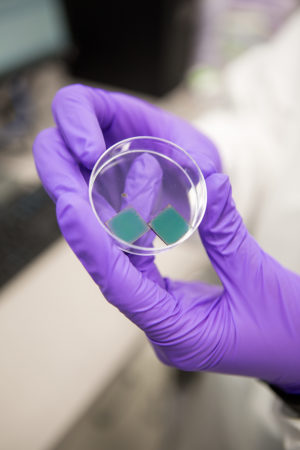
Last summer, before embarking on his 16th year teaching organic chemistry at North Seattle College, Jim Patterson worked with UW materials science & engineering professor Christine Luscombe to design an experiment where students could create their own solar cells.
Conventional solar panels rely on silicon crystals to absorb the sun’s light and convert it into electricity. High-quality silicon crystals are expensive and energy-intensive to make, so Luscombe’s research group is working on an alternative: photovoltaic polymers, which are plastic-like chains of molecules that can absorb visible light and convert that energy into electricity in a similar manner to silicon. These polymers can be made into thin, lightweight solar cells in conditions that are accessible to an undergraduate lab. During Patterson’s six weeks in the Luscombe lab, he took a crash course in making one of these photovoltaic polymers, known as poly-3-hexathiolphene (P3HT). Then, he learned how to layer the P3HT onto the base of a solar cell by studying an experiment that was designed by a previous Clean Energy RET participant, an instructor at Green River College.
“I felt like I got to go back to grad school,” said Patterson, who is a UW alum and former lab tech for the UW chemistry department. “Being exposed to Christine’s level of knowledge gave me a sense of the big questions on the cutting edge of this field, and I’m grateful for her mentorship. As I continue to develop the experiment, I’d feel more than comfortable popping in to ask a question. Her grad students like Lorenzo Guio and Wes Tatum were also indispensable resources for me, teaching me tricks and subtleties to the reaction that you can’t get from a textbook!”
Patterson has found success as an educator by making chemistry as hands-on as possible. In his course, students make soap, a few different hues of watercolor paint, and the same esters that give fruit candies their scents — all without advanced lab equipment. Synthesizing P3HT and layering it onto the device requires a more seasoned hand, so the solar cell project serves as a capstone to the course. Patterson knows the reward of seeing a handmade solar cell absorb light and produce current is worth the buildup.
“I’ve always felt compelled to put chemistry in context for my students,” he explained. “Ten years ago, I developed an experiment where students turned used french fry oil into biodiesel, and we talked about it in the context of oil and petroleum products. With this new lab, we talk about climate change and how solar panels can provide the energy of the future. I’m not just training scientists — I want to educate global citizens.”
Anthony Molinero, chemistry faculty at Grays Harbor College
Quantum dots for forensics
Tony Molinero, an instructor at Grays Harbor College in Aberdeen, WA, first heard about the RET program by recommending students for CEI’s adjacent Research Experience for Undergraduates (REU) program. After working for 18 years as a professor at the State University of New York at Potsdam, he had seen firsthand the value of immersing undergraduates in the cutting-edge science of a research lab. But since moving back to Washington state to take care of his elderly parents five years ago, he hadn’t had an opportunity to perform research himself.“The RET was the perfect way for me to learn about a new area of chemistry,” said Molinero. “I had a lot of fun getting back into the lab, and I was also able to bring an exciting new project back to my students!”
Molinero worked with chemistry professor Brandi Cossairt to develop a lab based on quantum dots, which are tiny particles — 10,000 times smaller than the width of a human hair — that can be engineered to have unique electronic and photonic properties. Cossairt’s research group studies quantum dots for applications like energy-efficient LED technology, solar energy, and alternative fuels. Some of these quantum dots are made from materials like cadmium selenide, which is toxic to humans in large quantities and unsafe for the lower-tech conditions of an undergraduate lab. One of Cossairt’s graduate students, Michael Enright, suggested the perfect alternative: a benign, carbon-based quantum dot that can be made in a microwave.
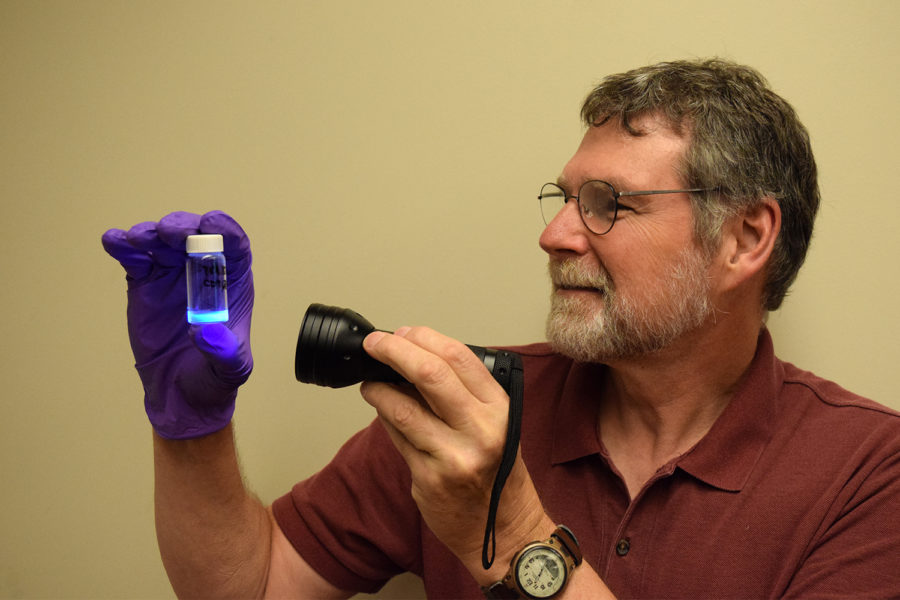
“Once we found a simple, safe quantum dot, we searched through the literature for inspiration,” explained Molinero. “We found an article in which scientists had derived similar carbon quantum dots from charred pig intestines. By embedding the quantum dots in a plastic, they could make permanent casts of fingerprints! It was a perfect application for my forensics and introductory chemistry classes at Grays Harbor.”
Molinero’s version of the quantum dot fingerprint cast only takes one to two class periods to make. After one hour of synthesis and then 30 to 40 minutes of purification from a viscous, brick-red solution, the result is illuminating for students: the quantum dots can take the shape of a dusted fingerprint, and shine a brilliant blue under an ultraviolet light. While the technology isn’t yet being used by field forensic scientists, it’s just one of many ways that Molinero hopes his students can explore quantum dots.
Some of Brandi’s students are using quantum dots for green applications like hydrogen fuel,” said Molinero. “While I didn’t have time to explore all of these reactions during my six weeks in her lab, I feel empowered to do so in the future!”