What is a perovskite?
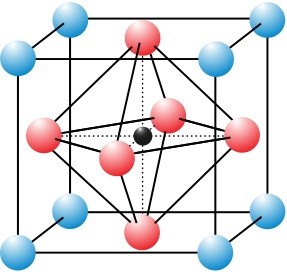
A perovskite is a material that has the same crystal structure as the mineral calcium titanium oxide, the first-discovered perovskite crystal. Generally, perovskite compounds have a chemical formula ABX3, where ‘A’ and ‘B’ represent cations and X is an anion that bonds to both. A large number of different elements can be combined together to form perovskite structures. Using this compositional flexibility, scientists can design perovskite crystals to have a wide variety of physical, optical, and electrical characteristics. Perovskite crystals are found today in ultrasound machines, memory chips, and now – solar cells.
Clean energy applications
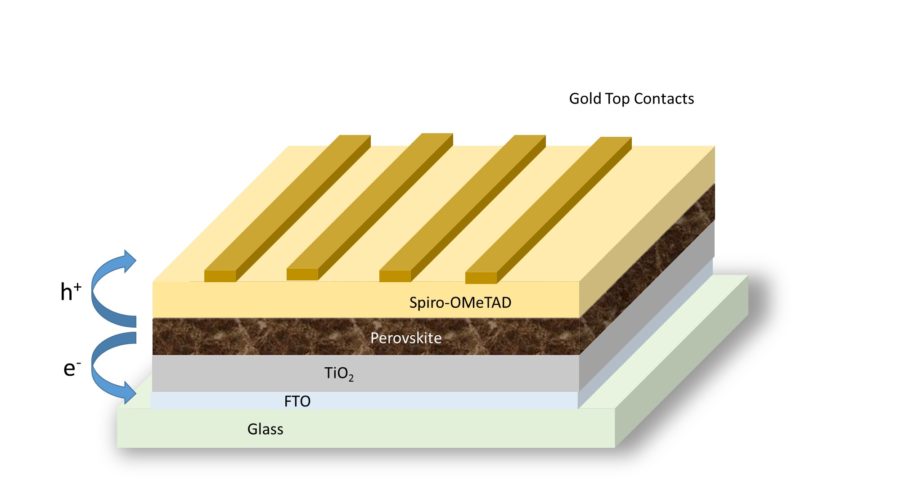
All photovoltaic solar cells rely on semiconductors — materials in the middle ground between electrical insulators such as glass and metallic conductors such as copper — to turn the energy from light into electricity. Light from the sun excites electrons in the semiconductor material, which flow into conducting electrodes and produce electric current.
Silicon has been the primary semiconductor material used in solar cells since the 1950s, as its semiconducting properties align well with the spectrum of the sun’s rays and it is relatively abundant and stable. However, the large silicon crystals used in conventional solar panels require an expensive, multi-step manufacturing process that utilizes a lot of energy. In the search for an alternative, scientists have harnessed the tunability of perovskites to create semiconductors with similar properties to silicon. Perovskite solar cells can be manufactured using simple, additive deposition techniques, like printing, for a fraction of the cost and energy. Because of the compositional flexibility of perovskites, they can also be tuned to ideally match the sun’s spectrum.
In 2012, researchers first discovered how to make a stable, thin-film perovskite solar cell with light photon-to-electron conversion efficiencies over 10%, using lead halide perovskites as the light-absorbing layer. Since then, the sunlight-to-electrical-power conversion efficiency of perovskite solar cells has skyrocketed, with the laboratory record standing at 25.2%. Researchers are also combining perovskite solar cells with conventional silicon solar cells – record efficiencies for these “perovskite on silicon” tandem cells are currently 29.1% (surpassing the record of 27% for conventional silicon cells) and rising rapidly. With this rapid surge in cell efficiency, perovskite solar cells and perovskite tandem solar cells may soon become cheap, highly efficient alternatives to conventional silicon solar cells.
Current research objectives
While perovskite solar cells, including perovskite on silicon tandems, are being commercialized by dozens of companies worldwide, there are still basic science and engineering challenges to address that can improve their performance, reliability, and manufacturability.
Some perovskite researchers continue to push conversion efficiencies by characterizing defects in the perovskite. While perovskite semiconductors are remarkably defect-tolerant, defects still negatively affect performance — especially those occurring at the surface of the active layer. Other researchers are exploring new perovskite chemical formulations, both to tune their electronic properties for specific applications (like tandem cell stacks), or further improve their stability and lifetime.
Researchers are also working on new cell designs, new encapsulation strategies to protect perovskites from the environment, and to understand basic degradation pathways so they can use accelerated aging studies to predict how perovskite solar cells will last on rooftops. Others are rapidly exploring a variety of manufacturing processes, including how to adapt perovskite “inks” to established large-scale solution printing methods. Finally, while the best-performing perovskites are today made with a small amount of lead, researchers are also exploring alternative compositions and new encapsulation strategies, in order to mitigate concerns associated with lead toxicity.
How is CEI advancing perovskites?
Perovskite crystals often exhibit atomic-scale defects that can reduce solar conversion efficiency. CEI Chief Scientist and chemistry professor David Ginger has developed “passivation” techniques, treating perovskites with different chemical compounds to heal these defects. But when perovskite crystals are assembled into solar cells, the current-collecting electrodes can create additional defects. In 2019, Ginger and collaborators at Georgia Tech received funding from the U.S. Department of Energy’s Solar Energy Technologies Office (SETO) to develop new passivation strategies and new charge collecting materials, allowing perovskite solar cells to reach their full efficiency potential while still remaining compatible with low-cost manufacturing.
Chemistry professor Daniel Gamelin and his group aim to modify silicon solar cells with perovskite coatings to collect high-energy photons of blue light more efficiently, bypassing the theoretical limit of 33% conversion for conventional silicon cells. Gamelin and his team have developed perovskite quantum dots — tiny particles thousands of times smaller than a human hair — that can absorb high-energy photons and emit twice as many low-energy photons, a process termed “quantum cutting.” Each photon absorbed by a solar cell generates one electron, so the perovskite quantum dot coating could dramatically increase conversion efficiencies.
Gamelin and his team have formed a spinoff company called BlueDot Photonics to commercialize the technology. With funding from SETO, Gamelin and BlueDot are developing deposition techniques to create thin films of perovskite quantum dots on the surface of conventional silicon solar cells, and prototyping large-area solar cells.
Chemical engineering professor Hugh Hillhouse is using machine learning algorithms to aid research of perovskites. Using photoluminescence captured by high-speed video, Hillhouse and his group are testing a variety of hybrid perovskites for long-term stability. These experiments generate enormous data sets, but by using machine learning, they aim to generate a predictive model of degradation for perovskite solar cells. This model can help them optimize the chemical makeup and structure of a perovskite solar cell for long-term stability — a key barrier to commercialization.
At the Washington Clean Energy Testbeds, an open-access lab facility operated by CEI, researchers and entrepreneurs can utilize state-of-the-art equipment to develop, test, and scale technologies like perovskite solar cells. Using the roll to roll printer at the Testbeds, perovskite inks can be printed at low temperatures onto flexible substrates. Testbeds technical director J. Devin MacKenzie, a professor of materials science & engineering and mechanical engineering at UW, is an expert on materials and techniques for high-throughput and low carbon-footprint manufacturing. One of his group’s most active projects, also funded by SETO, is developing in situ instruments that can measure the growth of perovskite crystals as they are being rapidly deposited during roll-to-roll printing. With support from the Joint Center for the Development and Research of Earth Abundant Materials (JCDREAM), MacKenzie’s group is also using the world’s highest resolution printer to develop new electrodes to pull electrical current out of perovskite solar cells without blocking sunlight from entering the cell.