The vanadium redox flow battery is a promising technology for grid scale energy storage. The tanks of reactants react through a membrane and charge is added or removed as the catholyte or anolyte are circulated. The large capacity can be used for load balancing on grids and for storing energy from intermittent sources such as wind and photovoltaics. The UET flow battery is the size of a shipping container and has 600kW power and 2.2MWh in capacity.

UniEnergy Flow Battery Source: http://www.uetechnologies.com/
The Chemistry
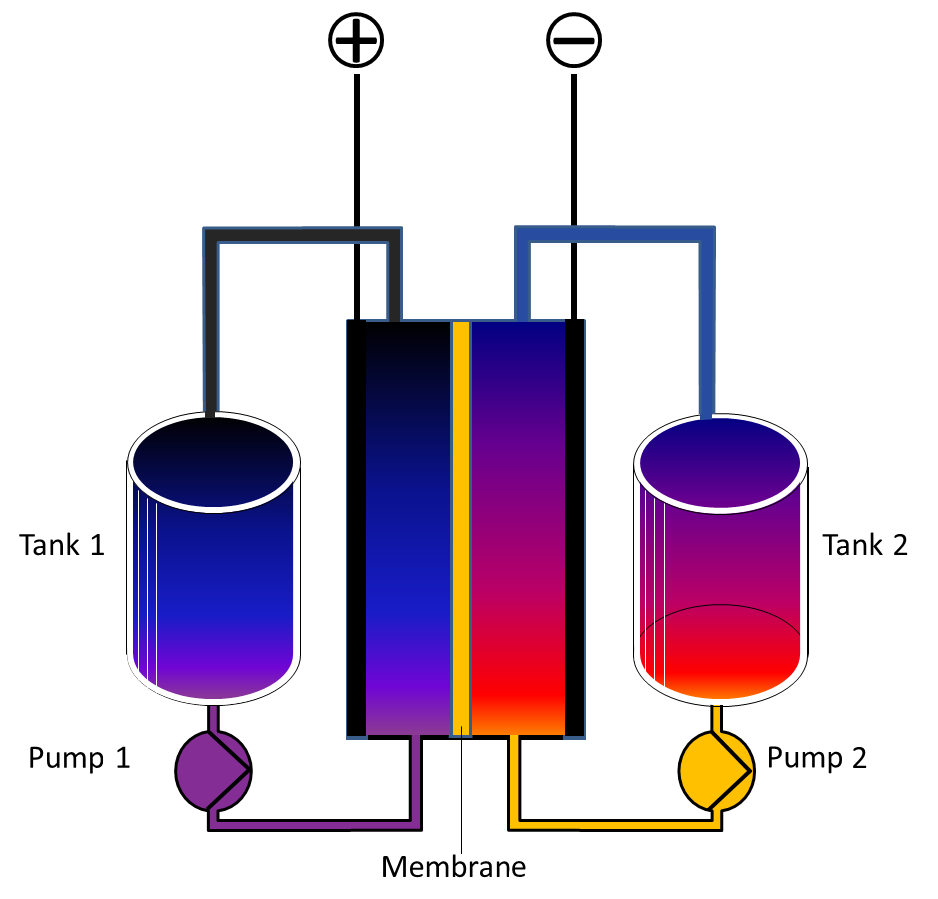
A flow battery consists of two tanks filled with chemicals in different oxidation states that react through a membrane. Charge is added or removed through two electrodes. Source: https://commons.wikimedia.org/wiki/File:Redox_Flow_Battery_English.png
One tank contains V5/4 mixture and the other contains a V2+/3+ mixture.
During discharge in the negative half cell V2+ is oxidized to V3+ and the electron that is freed travels to the external circuit to perform work. At the same time in the positive half cell V5+ is reduced to V4+ in the form of VO2+. H+ ions pass through the membrane to maintain charge balance.
catholyte
VO2+ +H2O <-> VO2+ + 2H+ +e–
anolyte
V3+ + e– <-> V2+
During charging each reaction is reversed. The charge state of the battery is described by the ratio of the species in each oxidation state in the two tanks. Power and energy are decoupled so either can be optimized. Power can be increased by changing the number or area of the membrane stacks so that more reactants can participate. Energy is controlled by the size of the storage tanks.
Links
UniEnergy Technology
http://www.uetechnologies.com/technology
Animation
Simplied animation





